NICE approves secukinumab for children with severe plaque psoriasis

NICE has published draft guidance recommending secukinumab for children aged 6 to 17 with severe plaque psoriasis.
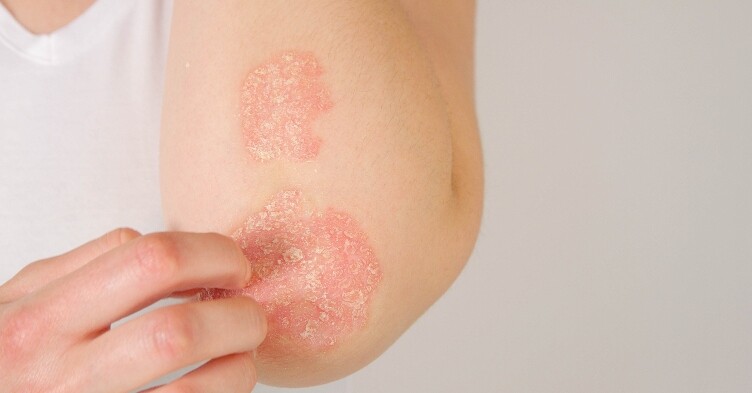
Marketed under the brand name Cosentyx and made by Novartis, secukinumab can be prescribed on the NHS for children and teens with severe plaque psoriasis, defined by a total psoriasis area and severity index (PASI) score of 10 or more.
Related Article: Call for regulatory guidelines as NHS adopts AI in dermatology care
It is recommended in patients whose disease has failed to respond to other systemic treatments, including ciclosporin, methotrexate and phototherapy, or who do not tolerate those treatments.
Plaque psoriasis is the most common type of psoriasis and affects up to 90% of people with the condition. Standard treatment for children and young people with the condition include topical ointments and lotions, followed by biologic treatments where appropriate.
Evidence has shown that secukinumab provides similar or greater overall health benefits than ustekinumab, etanercept and adalimumab.
Related Article: Abdominal body fat is a higher risk for developing psoriasis
Treatment should be halted at 12 weeks if the patient has not mounted an adequate response, defined as a PASI score reduction of 75%,
The final guidance is expected to be published next month.
Related Article: CPD: Case by case – acute and emergency dermatology presentations
This article was originally published on Nursing in Practice sister publication The Pharmacist.
To complete relevant dermatology CPD modules on Nursing in Practice Learning, click here.

See how our symptom tool can help you make better sense of patient presentations
Click here to search a symptom