Positive findings for drug treatment in young children with severe eczema
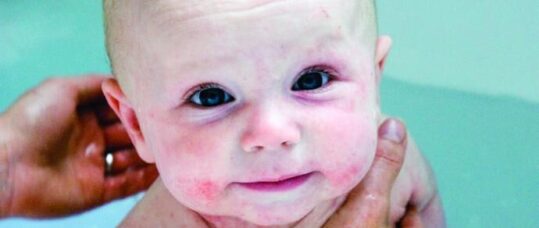
A biologic therapy has been shown to reduce eczema symptoms in infants and young children by 75% in a trial, and may offer an alternative to immune-suppressing medications for the age group.
The international study, which involved clinical scientists from the University of Manchester, has shown for the first time that the monoclonal antibody dupilumab can be safely and effectively used to reduce eczema and itching in children aged six months to five years.
The drug was found to significantly reduced the severity of the condition, decreasing skin itching and pain within two weeks. As a result of the treatment, young children with moderate-to-severe eczema could sleep through the night and have an improved quality of life, researchers found.
Related Article: Call for regulatory guidelines as NHS adopts AI in dermatology care
Although the therapy is already licensed in the UK for adults and children aged six to 18, dupilumab is not currently available for younger patients on the NHS.
The researchers describe the positive results as ‘the icing on the cake’ and published their findings in The Lancet.
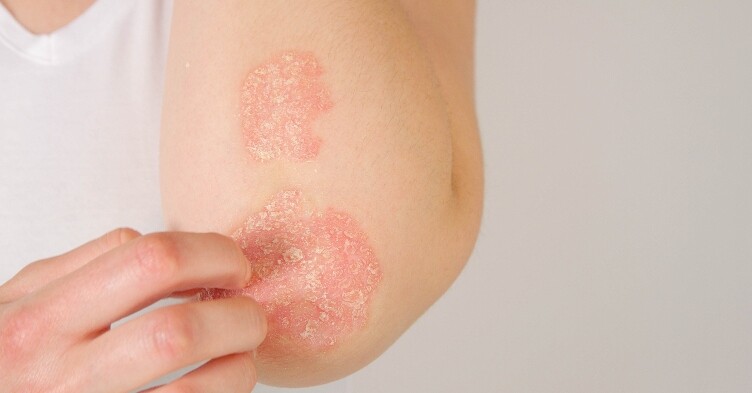
Eczema affects an estimated 20 percent or more of all children under six years of age. The chronic inflammatory skin condition is characterised by an itchy red rash, particularly on the face and the bends of the elbows and knees, and an increased risk of skin infection.
Most young children are treated with steroid creams and moisturisers. However, in one-third of cases, the itch can be debilitating, causing sleep disturbance and poor neurocognitive development, and alternative treatments are required.
Doctors expect that the international study of 162 patients will ensure this treatment is approved for British children shortly, following its adoption in the United States in June this year.
Related Article: Abdominal body fat is a higher risk for developing psoriasis
Dr Peter Arkwright, a senior lecturer at the University of Manchester and Consultant Paediatrician at the Royal Manchester Children’s Hospital, said: ‘We’re so delighted that dupilumab has provided clinically meaningful improvement, with an acceptable safety profile.’
During the study, children with moderate to severe eczema were enrolled from hospitals, clinics, and academic institutions in Europe and North America between June 2020 and February 2021. A total of 83 participants were given an injection of dupilumab under the skin, and 79 were given a placebo, every four weeks for sixteen weeks. All participants continued with steroid cream treatments during this time.
More than half of the children receiving dupilumab in the study had at least a 75% reduction in signs of eczema, as well as itch reductions and improved sleeping patterns. Over a quarter of participants on dupilumab had clear or almost clear skin by week 16.
Related Article: CPD: Case by case – acute and emergency dermatology presentations
Dr Arkwright said: ‘Young children and infants with moderate-to-severe eczema have a substantially reduced quality of life. It is also incredibly stressful for their families, particularly as children’s sleep is so disturbed. The fact that infants and young children with moderate-to-severe eczema are inadequately controlled with creams means they have a high unmet medical need.’

See how our symptom tool can help you make better sense of patient presentations
Click here to search a symptom