NICE issues final guidance for rheumatoid arthritis
A number of drugs for treating rheumatoid arthritis have been recommended today by the National Institute for Health and Care Excellence (NICE).
In final updated guidance NICE recommends that severe rheumatoid arthritis, which has not responded to intensive therapy, is treated with a combination of conventional biological disease modifying drugs (DMARDs).
Related Article: New preceptorship package for social care nurses
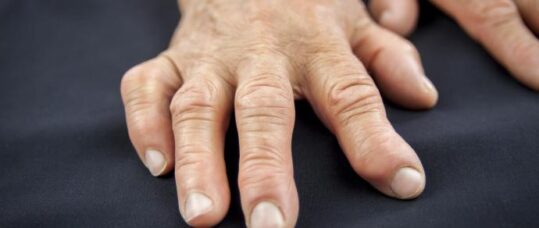
Rheumatoid arthritis is an incurable chronic systemic inflammatory autoimmune disease in which the synovial joints (such as those in the hands and feet) become inflamed, causing pain, swelling and stiffness. It’s associated with increased mortality and increasing disability and can have a severe effect on quality of life.
The disease affects around 400,000 people in the UK, of whom approximately 15% have severe disease. It is about 2-4 times more common in women than in men. It can develop at any age, but the peak age of onset in the UK is about 40-70 years.
The guidance states that treatment should be started with the least expensive drug (taking into account administration costs, dose needed and product price per dose).
Professor Carole Longson MBE, director of the Health Technology Evaluation Centre at NICE said: “This guidance considers at what stage it’s clinically and cost effective to start using biological therapies as treatment options for adults with rheumatoid arthritis.
Related Article: Applications to study nursing in England at ‘new low’
“In recommending them as options for people with severe rheumatoid arthritis after previous treatment with conventional DMARDs has been unsuccessful, this guidance reaffirms our previous recommendations on these drugs and confirms their place as an integral part of the rheumatoid arthritis treatment pathway,” she added.
The guidance also includes recommendations about when treatment with biological DMARDs should be continued or withdrawn.
Related Article: Paul Rees appointed as permanent NMC chief executive and registrar
Specifically, it recommends adalimumab (Humira, AbbVie), etanercept (Enbrel, Pfizer), infliximab (Remicade, Merck Sharp and Dohme; Inflectra, Hospira UK; Remsima, Napp Pharmaceuticals), certolizumab pegol (Cimzia, UCB Pharma), golimumab (Simponi, Merck Sharp and Dohme), tocilizumab (RoActemra, Roche) and abatacept (Orencia, Bristol-Myers Squibb), each in combination with methotrexate.
Adalimumab, etanercept, certolizumab pegol or tocilizumab are also recommended as monotherapy for people who cannot take methotrexate. In the case of certolizumab pegol, golimumab, abatacept and tocilizumab the recommendation is subject to the companies providing them as agreed in their patient access schemes.

See how our symptom tool can help you make better sense of patient presentations
Click here to search a symptom


NICE recommends that severe rheumatoid arthritis, which has not responded to intensive therapy, is treated with a combination of conventional biological disease modifying drugs



