Wegovy weight-loss injections approved by NHS
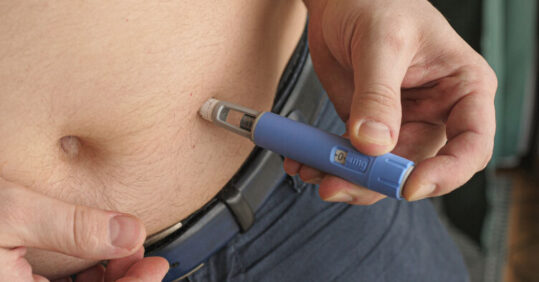
The weight-loss drug semaglutide – to be launched as Wegovy – will be available for prescription via the NHS following the recommendation of its use by the National Institute for Health Care and Excellence (NICE).
Patients inject themselves once a week with pens prefilled with semaglutide. The drug suppresses appetite by mimicking the hormone glucagon-like peptide-1 (GLP-1), which is released after eating. It makes people using it feel full, thereby resulting in people eating less and reducing their overall calorie intake.
Related Article: NHS 10-year plan: What does it mean for nursing?
NICE has recommended use of semaglutide, alongside a reduced-calorie diet and increased physical activity, for adults who have at least one weight-related comorbidity and a body mass index (BMI) of at least 35kg/m2. A weight-related comorbidity could be one of: dysglycaemia (prediabetes or type 2 diabetes mellitus); hypertension; dyslipidaemia (in which disturbances in fat metabolism lead to changes in the concentrations of lipids in the blood); obstructive sleep apnoea; or cardiovascular disease.
NICE said semaglutide will be available to NHS patients when the launch of the drug in England is confirmed by manufacturer Novo Nordisk. A spokesperson for the company said: ‘I can confirm that we don’t have a launch date for Wegovy, but are still working to make it available as soon as possible in the UK’.
While NICE said that Wegovy would be available via the NHS specialist weight management service, Boots is among several online providers intending to offer the drug subject to assessment and prescription via an online GP, and interested parties can already sign up for information via the company’s website.
However, Helen Knight, director of medicines evaluation at NICE, emphasised that semaglutide ‘won’t be available for everyone’.
According to NICE’s recommendation, the drug can only be prescribed for a maximum of two years within a specialist weight management service providing multidisciplinary management of overweight or obesity.
Related Article: Funded nurse workforce plan needed for neighbourhood health services
Ms Knight explained: ‘Our committee has made specific recommendations to ensure it remains value for money for the taxpayer, and it can only be used for a maximum of two years.
‘For some people, losing weight is a real challenge, which is why a medicine like semaglutide is a welcome option.’
Another self-injected medication manufactured by Novo Nordisk, Ozempic – a once-weekly injection of semaglutide indicated for the management of type 2 diabetes – attracted headlines recently when Jeremy Clarkson wrote a column for The Times revealing he had received it off licence.
Related Article: Over one million children living in homes causing asthma and chronic illness
This, along with a range of TikTok influencers claiming to be using the drug in the UK, has helped to create an increase in demand for Ozempic that has, in turn, put ‘enormous pressure’ on the supply chain for type 2 diabetes.
Saxenda (liraglutide), a weight-loss injection, used daily, also manufactured by Novo Nordisk, was approved for use in the UK in October 2021.

See how our symptom tool can help you make better sense of patient presentations
Click here to search a symptom




